Abstract
Background: Invasive fungal infections (IFI) can occur up to 40% of patients with hematologic malignancies and are most common in patients with acute myeloid leukemia (AML). National averages within the United States, per Infectious Diseases Society of America (IDSA), range approximately from 8-10% in this high risk population. IFIs are not only problematic for individual patient care, potentially resulting in increased antimicrobial usage and delay of future chemotherapy treatments or stem cell transplant, but IFI also negatively impact hospital resource utilization including increased days in hospital and the intensive care unit (ICU).
Methods: In attempt to decrease IFIs in hematologic malignancy patients at the University of Virginia Health System (UVAHS), a multidisciplinary team of oncology and infectious diseases physicians, nurses, and pharmacists was formed to develop an Antifungal Program. Retrospective chart review was done of 79 patient encounters from 7/2011 to 9/2014 to establish our baseline cohort. For each of these encounters, extensive patient demographics, clinical, and inpatient event data were collected. Analysis of our baseline data showed a 19.7% proven or probable IFI rate in patients with acute myeloid leukemia undergoing induction chemotherapy. In concordance with IFI rates published by IDSA, we aimed to decrease the IFI in this patient population at UVAHS to less than 10%. With guidance from the American Society of Clinical Oncology's Quality Training Program, our team utilized multiple qualitative and quantitative tools to further understand factors contributing to an increased rate of IFIs. These tools included a process map and Ishikawa cause-and-effect diagram to depict all the steps from leukemia diagnosis to end of treatment and hospital discharge, a Pareto chart to quantify delays of individual steps, and a priority matrix categorizing potential interventions based on impact and ease of implementation. A statistical process control chart (p-chart) depicted rates of IFI in the baseline cohort and intervention groups.
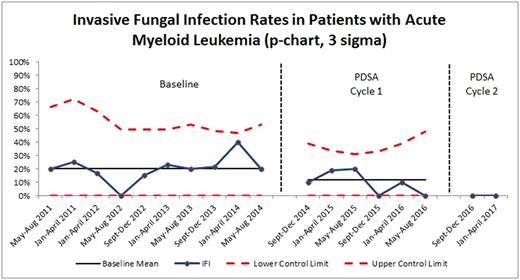
Results: Our baseline proven or probable IFI rate in patients with acute myeloid leukemia was 19.7%. Antifungal prophylaxis was not administered to baseline cohort patients. Per Pareto chart analysis, the team identified the most common problems contributing to IFI: inconsistent definition of IFI, no routine antifungal prophylaxis, and lack of education for the health care team. Two Plan-Do-Study-Act (PDSA) cycles of interventions were implemented; post-intervention data were collected and analyzed. From 9/2014 to 8/2016, the first PDSA cycle consisted of individual attending physicians ordering antifungal prophylaxis on an ad hoc basis; the first PDSA cycle resulted in a decreased IFI rate of 11.7% (n = 77). Though close to project aim of less than 10%, this process was burdensome and not sustainable, as it required individual physicians to remember to add antifungal prophylaxis and did not standardize which antifungal agent was used. During the second PDSA cycle from 8/2016 to 4/2017, a clinical practice guideline standardized the definition of IFI and recommended posaconazole as the prophylactic antifungal of choice. Team champions educated physicians, nurses, and pharmacists on the new standard work. This second PDSA cycle resulted in zero IFIs (n = 18), surpassing the team's goal of improving IFI to less than 10%. P-chart depicting IFI reduction is shown in Figure 1. Additionally, median ICU length of stay improved from 2.5 days to 1.5 days. Overall hospital length of stay decreased from 32.3 days to 29.9 days. As no proven or probable IFI were diagnosed during PDSA Cycle 2, no resistant fungal organisms were noted.
Conclusions: This project illustrates the importance of forming a multidisciplinary team, whose collaborative effort developed an Antifungal Program that reduced IFI rates in a statistical significant method. When compared to historical controls, the Antifungal Program decreased both IFI rates and ICU length of stay. To ensure sustainability of the quality improvement study, monthly electronic medical record reports will capture guideline compliance, specifically the use of antifungal agents, and track future IFI. The Antifungal Program is an effective clinical tool to not only improve patient care, but likely represents increased patient quality of life, cost-savings, and decreased length of stay.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal